When your doctor talks about switching from a brand-name drug to something cheaper, you might hear the words biosimilar or generic. At first glance, they sound like the same thing: a lower-cost version of a medicine you’ve been taking. But they’re not. And confusing them could cost you more than just money-it could affect how well your treatment works.
What’s the Real Difference?
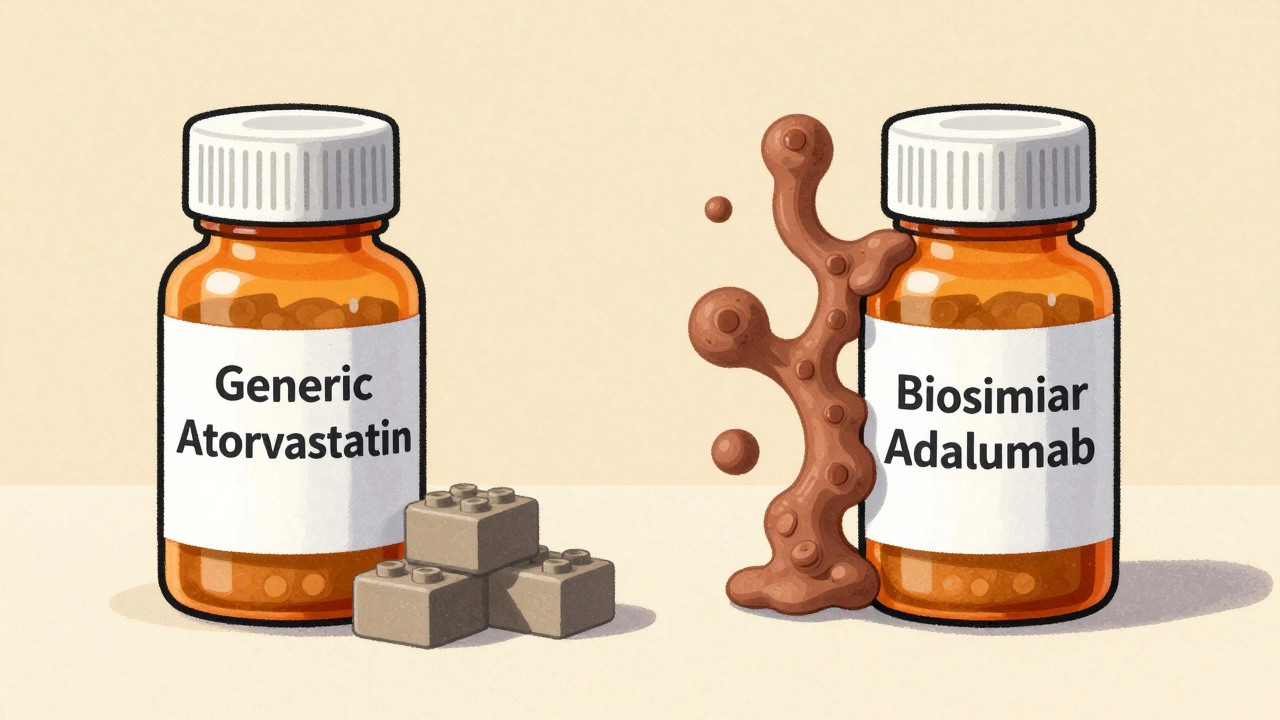
Generics and biosimilars both save money, but they come from completely different worlds of science.Generics are copies of small-molecule drugs. These are pills or injections made from simple chemical formulas you can recreate exactly in a lab. Think of them like copying a Lego brick: you use the same pieces, same shape, same color. If you’ve taken atorvastatin instead of Lipitor, or levothyroxine instead of Synthroid, you’ve used a generic. The FDA requires these to be identical in active ingredient, strength, dosage form, and how your body absorbs them. That’s why they’re considered interchangeable by default in most states.
Biosimilars, on the other hand, are copies of biologic drugs-large, complex proteins made from living cells. These aren’t built in a flask like chemicals. They’re grown in labs using living organisms like hamster or yeast cells. Even tiny changes in temperature, pH, or nutrient mix can alter the final product. That’s why a biosimilar isn’t identical to its reference drug. It’s highly similar, with no clinically meaningful differences in safety or effectiveness. Think of it like making a replica of a handmade sculpture: you get something that looks and functions almost the same, but the brushstrokes aren’t perfectly matched.
Cost Savings: Big Difference, Big Impact
Generics slash prices by 80-85%. A brand-name cholesterol pill that costs $200 a month? The generic might be $30. That’s life-changing for people on fixed incomes.Biosimilars? They save 15-20%. That sounds less impressive until you realize the original biologic costs $10,000 to $20,000 a year. A $2,000 savings still means thousands less out of pocket. For someone on a biologic for rheumatoid arthritis or cancer, that’s not a small win-it’s access to treatment they couldn’t otherwise afford.
And here’s the catch: you can’t get a generic version of a biologic. Ever. Because the molecule is too complex to copy exactly. That’s why biosimilars exist-to bring down the cost of these high-priced, life-saving drugs.
How Are They Approved?
Generics don’t need new clinical trials. The FDA just checks that they work the same way in your body as the brand drug-measuring blood levels over time. If the numbers fall within 80-125% of the original, it’s approved. Simple. Fast. Cheap. Development costs around $2-3 million.Biosimilars? They need years of work. Manufacturers must show:
- Extreme similarity in molecular structure (using advanced tools like mass spectrometry)
- Same biological activity in lab tests
- Comparable safety and effectiveness in clinical trials
- No increased risk of immune reactions
That’s why development costs $100-250 million and takes 8-10 years. The FDA doesn’t just look at one thing-they look at the totality of evidence. It’s not about proving they’re identical. It’s about proving they’re just as safe and effective.
Can Pharmacists Switch Them Automatically?
With generics? In 49 states, yes. If your doctor doesn’t write “dispense as written,” your pharmacist can swap the brand for the generic without asking you. It’s built into the system.With biosimilars? It’s not that simple. Only biosimilars labeled “interchangeable” can be swapped without the prescriber’s permission. And even then, 28 states require the pharmacist to notify your doctor within 72 hours. Why? Because biologics can trigger immune responses. Even if the risk is tiny, regulators want to track every switch.
And here’s a real-world problem: many biosimilars come in different delivery devices. A Humira biosimilar might use a pen that looks and feels different from the original. Elderly patients or those with arthritis have reported confusion-sometimes even dosing errors. It’s not the drug’s fault. It’s the packaging.

Are They Safe? Real-World Data
A 2019 JAMA study looked at 47 trials comparing generics to brand-name heart drugs. Result? No difference in heart attacks, strokes, or deaths. Generics are proven.For biosimilars, the evidence is strong too. A 2022 review of 128 studies on infliximab (used for Crohn’s and rheumatoid arthritis) tracked over 38,000 patients. No increase in side effects or treatment failure when switching to the biosimilar.
Still, some patients worry. A 2022 survey by the National Psoriasis Foundation found 42% of patients were hesitant to switch. One patient on Reddit said, “I was scared my skin flare-up would come back.” But after switching to a biosimilar adalimumab, her symptoms stayed under control-and her co-pay dropped from $400 to $80.
The FDA’s adverse event database shows biosimilars have nearly identical safety profiles to their reference products. For infliximab, there were 0.12 adverse events per 100 patient-years with the biosimilar versus 0.15 with the original. Not statistically different.
Where Are They Used?
Generics are everywhere: blood pressure pills, antibiotics, antidepressants, thyroid meds. If it’s a small molecule, there’s likely a generic.Biosimilars? They’re in specialty areas:
- Oncology: Trastuzumab for breast cancer, bevacizumab for colon cancer
- Autoimmune diseases: Adalimumab for rheumatoid arthritis, infliximab for Crohn’s
- Diabetes: Insulin glargine (Semglee, the first interchangeable biosimilar insulin)
These are drugs that used to cost tens of thousands a year. Now, biosimilars are making them accessible. In the U.S., 83% of filgrastim (a cancer drug that boosts white blood cells) prescriptions are now for biosimilars.
What’s Holding Back Biosimilars?
You’d think with all the savings, everyone would jump on board. But there are barriers:- Patent lawsuits: Drugmakers file dozens of patents to delay biosimilar entry. One biologic can have over 140 patent claims. This delays market access by nearly 5 years on average.
- Prescriber confusion: A 2023 AMA survey found only 58% of non-specialist doctors felt confident prescribing biosimilars. Many still think they’re “less safe.”
- Insurance formularies: Some plans still favor the original brand because of rebates or contracts.
- Patient fear: Even when data says it’s safe, anxiety sticks. “What if it doesn’t work for me?” is a real concern.
But things are changing. The Inflation Reduction Act of 2022 removed financial penalties for doctors who prescribe biosimilars in Medicare. That’s pushing adoption. By 2027, biosimilars could make up 45% of all biologic prescriptions.
How to Decide What’s Right for You
Ask these questions:- Is this a small-molecule drug? If yes, a generic is your best-and safest-bet.
- Is it a biologic? If yes, a biosimilar is your only affordable option.
- Has my doctor explained why they’re suggesting this switch? Don’t be afraid to ask: “Is this a biosimilar or a generic? How do you know it’s safe for me?”
- What’s my insurance coverage? Sometimes, even if a biosimilar is cheaper, your plan might still require you to try the brand first.
- Do I have a history of immune reactions? For some conditions like inflammatory bowel disease, your doctor might prefer to stick with the original biologic until more long-term data is available.
Don’t assume cheaper means riskier. The science says otherwise. But do make sure you understand what you’re taking-and why.
Where to Find Reliable Info
The FDA’s Orange Book lists all approved generics. The Purple Book lists biosimilars and interchangeable products. Both are free to search online. Your pharmacist can help you look them up.Also, many biosimilar manufacturers offer patient support programs. Amgen, Sandoz, and Pfizer all have services that help with prior authorizations, co-pay assistance, and even one-on-one nurse education.
And if you’re unsure? Talk to your doctor or pharmacist. Don’t let fear or confusion stop you from getting the treatment you need. The goal isn’t just to save money. It’s to get you the best care possible-without breaking the bank.
Are biosimilars as safe as the original biologic drugs?
Yes. The FDA requires biosimilars to show no clinically meaningful differences in safety, purity, or potency compared to the reference product. Thousands of patients have been studied in clinical trials and real-world use, and data consistently show similar rates of side effects and treatment success. For example, biosimilar infliximab has the same safety profile as the original, with only 0.12 adverse events per 100 patient-years versus 0.15 for the reference.
Can I be switched from a brand-name biologic to a biosimilar without my doctor’s approval?
Only if the biosimilar is labeled “interchangeable” and your state allows pharmacy-level substitution. Even then, 28 states require the pharmacist to notify your doctor within 72 hours. If the biosimilar isn’t interchangeable, your doctor must specifically prescribe it. Never assume a switch will happen automatically with biologics.
Why are biosimilars more expensive than generics?
Because they’re far more complex to make. Generics are simple chemicals you can replicate exactly. Biosimilars are large proteins made from living cells, requiring years of research, advanced testing, and specialized manufacturing. Development costs for a biosimilar average $100-250 million-50 to 100 times more than a generic.
Do biosimilars cause more side effects or immune reactions?
No. Studies tracking over 38,000 patients on biosimilar infliximab found no increase in immune reactions or treatment failure compared to the original. While biologics can trigger antibodies, this risk exists with both the reference product and biosimilars. The FDA requires manufacturers to prove their product doesn’t increase this risk.
Can I switch back to the brand-name drug if I don’t feel well on a biosimilar?
Yes. If you experience any new or worsening symptoms after switching, talk to your doctor. There’s no rule that says you have to stay on the biosimilar. Many patients switch back successfully. The key is communication-don’t stop or change your medication without medical advice.
Are there any drugs that can’t be replaced with biosimilars or generics?
Some drugs have no generic or biosimilar version yet because the patent hasn’t expired. Others, like certain narrow-therapeutic-index drugs (e.g., warfarin, lithium), are sometimes kept as brand-name due to concerns about small variations in blood levels. But for most conditions-especially chronic diseases like arthritis, cancer, or diabetes-biosimilars and generics are safe, effective alternatives.
What’s Next?
The landscape is shifting fast. More biosimilars are coming-especially for drugs like Stelara and Humira, which are among the top-selling biologics in the world. By 2028, biosimilars could save the U.S. healthcare system over $34 billion.But progress depends on education. Patients need to understand the science. Doctors need better training. Pharmacists need clearer guidelines. And insurers need to stop favoring expensive brands just because they’re profitable.
Choosing between a biosimilar and a generic isn’t about picking the cheapest option. It’s about picking the right one for your body, your condition, and your life. Ask questions. Do your research. And trust the science-it’s on your side.

Irving Steinberg
Generics are the real MVPs 🙌 I switched my blood pressure med to generic and saved $180 a month. No side effects, no drama. Why are people still scared of them? 😅
Lydia Zhang
Biosimilars are just expensive generics with extra steps
Kay Lam
I want to take a moment to recognize how important it is that we’re having this conversation at all. So many people are terrified to switch medications because they don’t understand the science and that fear is completely valid even if the data says otherwise. The FDA doesn’t approve these drugs lightly and the clinical trials for biosimilars are some of the most rigorous in all of pharmacology. It’s not just about cost-it’s about access. When someone with rheumatoid arthritis can afford to keep treating their pain instead of choosing between rent and their biologic, that’s a win for humanity. And yes the packaging changes can be confusing but that’s a logistics problem not a safety one. We need better patient education not more fear.
Matt Dean
You people are still debating this? The science is settled. If you’re still using brand-name insulin in 2025 you’re either rich or dumb. Biosimilars have been out for a decade. The data is clear. Stop being a liability to the healthcare system.
Walker Alvey
So we’re paying $250 million to make a copy that’s ‘highly similar’ but not identical? And we call this innovation? The system is a joke. Big Pharma invented the word biosimilar so they could keep charging $10K a year and still get to say ‘we’re lowering costs’ while pocketing the difference. The real biosimilar is the one that breaks the patent monopoly
Adrian Barnes
The data presented here is statistically insignificant and methodologically flawed. The JAMA study cited has a sample size too small to infer population-level safety. Furthermore, the adverse event reporting system is notoriously underutilized and biased toward acute reactions. Long-term immunogenicity data for biosimilars remains incomplete. Until we have 15-year longitudinal studies, prescribing these agents constitutes a clinical gamble.
Declan Flynn Fitness
Big fan of biosimilars here. My cousin switched from Humira to the biosimilar and her flare-ups actually improved. The pen felt weird at first but her nurse walked her through it. Saved her $300/month. Life-changing. 🙏
Linda Migdal
America leads the world in biologic innovation and now we’re letting foreign manufacturers copy our life-saving drugs? This is economic surrender. We need tariffs on biosimilar imports and subsidies for domestic biologics. This isn’t healthcare-it’s national security
Shannon Gabrielle
So the FDA says biosimilars are safe but the packaging is different and the pen clicks louder? Sounds like a lawsuit waiting to happen. Someone’s gonna mix up doses and then we’ll get the 20/20 hindsight documentary about how the system failed grandma
Eric Vlach
I’ve worked with patients on biologics for over a decade and the biggest barrier isn’t science-it’s trust. People need to hear from someone who’s been there. I always tell them: if your doctor says it’s safe and the FDA says it’s safe and your pharmacist says it’s safe, then it’s safe. And if you’re still nervous? Try it for 3 months. If nothing changes, you can always go back. No one’s taking your life away. You’re just saving money while staying healthy
Souvik Datta
In India, we’ve seen how generics transformed diabetes care. Now we’re watching biosimilars do the same for cancer. The real question isn’t whether they work-it’s whether our systems are ready to deliver them. Education, supply chains, and trust matter more than the molecule itself. Science is universal. But access? That’s a human problem
Priyam Tomar
Everyone’s acting like biosimilars are some new miracle. Newsflash: they’ve been used in Europe since 2006. The US is 15 years behind. And now we’re acting shocked that patients are scared? You think the problem is patients? No. The problem is doctors who still think generics are ‘cheap knockoffs’ and insurance companies that reward brand loyalty. Fix the system not the patients
Jack Arscott
My dad switched to a biosimilar for his arthritis and now he’s hiking again 🥳 Thank you to everyone who made this possible. I’m just glad we’re talking about it.