Canagliflozin Amputation Risk Assessment
Personal Risk Assessment
This tool helps you understand your risk of lower-limb amputation while taking canagliflozin based on medical factors. According to guidelines, having two or more risk factors significantly increases your risk.
When you’re managing type 2 diabetes, finding the right medication isn’t just about lowering blood sugar. It’s about balancing benefits with real, sometimes scary, risks. One drug that’s sparked intense debate is canagliflozin, sold under the brand name INVOKANA®. It works well-helping many people drop their A1c levels and lose weight. But it’s also linked to a rare but serious risk: lower-limb amputation.
What Does the Evidence Actually Say?
The concern didn’t come out of nowhere. In 2017, the CANVAS Program, a large study combining two major clinical trials, found that people taking canagliflozin had nearly twice the risk of needing an amputation compared to those on placebo. The numbers were clear: 5.5 amputations per 1,000 patient-years for the 300 mg dose, versus 2.8 for placebo. That’s not a small increase. Most of these were minor amputations-toes or parts of the foot-not full leg removals. But even one toe amputation can change your life. The FDA responded fast. They added a boxed warning-the strongest kind-to the drug label. For a while, many doctors stopped prescribing it. But then more data came in. The CREDENCE trial, which focused on patients with diabetic kidney disease, showed that canagliflozin significantly reduced heart failure and kidney failure. The benefits were too big to ignore. In 2020, the FDA removed the boxed warning-but didn’t remove the risk. It’s still clearly listed in the Warnings and Precautions section of the prescribing info. Here’s what’s critical to understand: this risk doesn’t seem to apply to all SGLT2 inhibitors. Drugs like empagliflozin (Jardiance) and dapagliflozin (Farxiga) haven’t shown the same pattern. A 2023 meta-analysis of over 74,000 patients confirmed that only canagliflozin had a statistically significant increase in amputation risk. That’s not a class effect-it’s specific to this one drug.Who’s at the Highest Risk?
Not everyone on canagliflozin will have problems. But some people are far more vulnerable. If you have any of these, your risk goes up:- Peripheral artery disease (PAD)-about 1 in 5 people with type 2 diabetes have this
- Diabetic neuropathy-nerve damage that makes your feet feel numb
- History of foot ulcers or prior amputations
- Smoking
- Absent or weak pulses in your feet
Why Does This Happen?
We don’t have a full answer yet, but researchers have strong theories. Canagliflozin lowers blood pressure more than other drugs in its class-by about 3.7 mmHg systolic on average. It also causes more weight loss-around 2.8 kg. That might sound good, but in someone with poor circulation, it can reduce blood flow even further to the feet. Add in the fact that SGLT2 inhibitors increase urine output, which can lead to mild dehydration, and you’ve got a recipe for slower healing. There’s also the issue of sensation. If you can’t feel a blister or cut because of nerve damage, and your blood flow is already low, that small injury can turn into an infection, then an ulcer, then an amputation. It’s not the drug alone-it’s the combination with pre-existing damage.
Real Stories, Real Consequences
Behind the statistics are real people. On PatientsLikeMe, nearly 7% of users reported foot problems. Seventeen people specifically mentioned amputation concerns. One Reddit user, u/DiabetesWarrior2020, shared that after 18 months on INVOKANA, he developed a non-healing ulcer that led to a toe amputation. His endocrinologist immediately switched him to Jardiance. But not everyone has bad experiences. Another user, u/SugarFreeLife, said she’s been on canagliflozin for three years with no foot issues and her A1c dropped from 8.5% to 6.2%. That’s the balance: for some, the benefits far outweigh the risks. For others, the risk is too high. The FDA’s adverse event database shows something telling: there were 1,892 amputation reports for canagliflozin out of 4.2 million prescriptions-that’s 0.045%. For empagliflozin, it was just 0.0026%. The odds of reporting an amputation were nearly 18 times higher with canagliflozin.How to Stay Safe If You’re Taking It
If you’re already on canagliflozin, don’t panic. But do take action.- Check your feet every day. Look for redness, swelling, cuts, blisters, or sores. Use a mirror if you can’t see the bottom of your feet.
- Report any pain or new sores immediately. Don’t wait. Even if it feels minor, get it checked the same day.
- Ask for a foot exam at every doctor visit. Your provider should check your pulses, sensation, and skin condition.
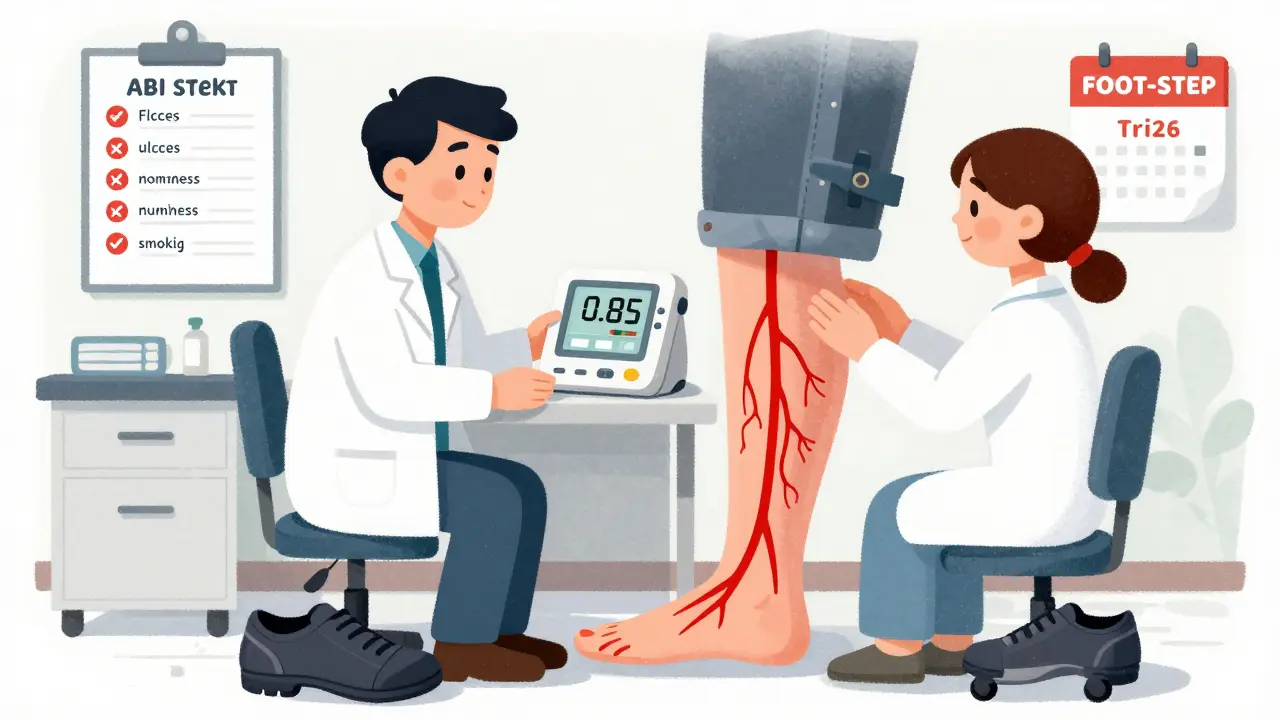
- Get an ankle-brachial index (ABI) test. The American Diabetes Association now recommends this before starting canagliflozin if you have any cardiovascular risk factors. An ABI under 0.9 means poor blood flow and is a red flag.
- Don’t smoke. Smoking narrows blood vessels and makes everything worse.
- Wear proper shoes. No barefoot walking, even at home. Check inside your shoes for pebbles or rough seams.
What Are Your Alternatives?
You don’t have to stay on canagliflozin if you’re worried. Other SGLT2 inhibitors-like empagliflozin and dapagliflozin-have proven cardiovascular and kidney benefits without the same amputation risk. In fact, dapagliflozin showed a trend toward fewer amputations in trials. Other diabetes medications also offer strong protection:- GLP-1 agonists like semaglutide (Wegovy, Ozempic) and liraglutide (Victoza) reduce heart attacks, strokes, and kidney disease-and have no amputation signal.
- Metformin remains first-line for most people and has decades of safety data.
- DPP-4 inhibitors like sitagliptin are neutral on amputation risk.
The Bigger Picture
Despite the concerns, canagliflozin is still prescribed. In 2023, it brought in $1.87 billion in global sales. It’s still on the WHO’s list of essential medicines-with a footnote about foot monitoring. That tells you something: it’s valuable, but only when used carefully. Prescribing patterns have shifted. In 2017, it was the most prescribed SGLT2 inhibitor. By 2024, its share dropped from 35% to 22%. But it stabilized. Doctors aren’t avoiding it-they’re being smarter. More prescriptions now come with the mandatory medication guide explaining the risk. In 2023, 68% of new prescriptions included it, up from 42% in 2017. And there’s hope on the horizon. Janssen is testing a new extended-release version (INVOKANA XR) that may reduce peak drug levels-and possibly lower the amputation risk. The FOOT-STEP trial, ending in 2026, is testing whether structured foot care programs can prevent amputations in high-risk patients.Bottom Line: It’s Not About Avoiding the Drug-It’s About Using It Wisely
Canagliflozin isn’t dangerous for everyone. But it’s dangerous for some-and we know who those people are. If you have healthy feet, good circulation, and no history of ulcers, it can be a powerful tool. If you have neuropathy, PAD, or prior foot problems, it’s not the right choice. The key isn’t fear. It’s awareness. Ask your doctor: “Do I have any risk factors for foot problems?” Get your feet checked. Know the signs. Speak up early. That’s how you protect yourself-not by avoiding treatment, but by using it with eyes wide open.Is canagliflozin still prescribed today?
Yes, canagliflozin is still prescribed, but less often than in 2017. Its use has dropped from 35% to 22% of SGLT2 inhibitor prescriptions as of 2024. Doctors now use it more selectively-mainly for patients with type 2 diabetes who have heart or kidney disease, no foot complications, and no history of poor circulation. The FDA removed its boxed warning in 2020 after reviewing additional data, but the amputation risk remains listed in the drug’s safety information.
Do all SGLT2 inhibitors cause amputation risk?
No. The increased amputation risk is specific to canagliflozin. Studies on empagliflozin (Jardiance) and dapagliflozin (Farxiga) show no significant increase in amputation risk. In fact, dapagliflozin showed a slight trend toward fewer amputations. This means the risk is not a class-wide effect but tied to canagliflozin’s unique pharmacological profile, possibly due to its stronger effects on blood pressure and weight loss.
How common are amputations with canagliflozin?
Amputations are rare but real. In clinical trials, the rate was about 5.5 events per 1,000 patient-years for the 300 mg dose. That means roughly 1 in 200 people taking the drug for one year might experience an amputation. The absolute increase is small-about 1.8 extra amputations per 1,000 people per year-but the impact is severe. Most are minor (toes or metatarsals), but even those can lead to long-term mobility issues.
What should I do if I notice a sore on my foot while taking canagliflozin?
Act immediately. Don’t wait. Even a small blister or red spot can become serious if you have nerve damage or poor circulation. Contact your doctor or podiatrist the same day. If you can’t reach them, go to an urgent care center or emergency room. Early treatment-cleaning, antibiotics, or offloading pressure-can prevent infection and amputation. Never ignore foot changes while on this medication.
Should I stop taking canagliflozin if I’m worried?
Don’t stop on your own. Talk to your doctor first. Stopping suddenly can cause your blood sugar to spike, which is dangerous. Your provider can assess your risk factors-like foot health, circulation, and kidney function-and help you decide whether to switch to another medication like empagliflozin or dapagliflozin, which don’t carry the same amputation risk but offer similar benefits.
Are there tests to check if I’m at risk before starting canagliflozin?
Yes. The American Diabetes Association now recommends an ankle-brachial index (ABI) test before starting canagliflozin if you have any cardiovascular risk factors. An ABI below 0.9 indicates poor blood flow in the legs and is a relative contraindication. Your doctor should also check for foot ulcers, pulses, and sensation during a comprehensive foot exam. These simple tests can prevent serious complications.

Ben Harris
So let me get this straight - a drug that helps your blood sugar and makes you lose weight also nukes your toes? And we’re supposed to be okay with that because the FDA says it’s fine now? I mean sure if you’re rich enough to afford a prosthetic toe and a lifetime of podiatry bills but for the rest of us it’s just another corporate gamble on our bodies
Justin James
Have you ever stopped to think that this whole amputation scare might be a distraction? The pharmaceutical industry has been pushing SGLT2 inhibitors hard for years because they’re profitable but the real issue is that diabetes care in this country is a complete mess - hospitals don’t do foot exams, primary care docs are overworked and don’t have time to check pulses, insurance won’t cover ABI tests unless you’re already missing toes, and then when someone loses a foot they blame the drug instead of the system that let it happen - it’s not canagliflozin that’s dangerous it’s the fact that we treat diabetes like a checklist and not a living condition and if you think this is the first time a drug got pulled because of a side effect only to come back with a new label you’re not paying attention to the last 30 years of pharma history
Zabihullah Saleh
It’s funny how we treat medicine like it’s a binary choice - take it or don’t - when really it’s about context. Canagliflozin isn’t evil. It’s a tool. Like a chainsaw. You wouldn’t hand one to someone who doesn’t know how to use it or who’s standing on unstable ground. But if you’re trained, have the right gear, and know your limits? It can cut through problems you didn’t think were fixable. The problem isn’t the drug. It’s that we hand out powerful tools like they’re candy and then act shocked when someone gets hurt. Maybe we need to stop asking if the drug is safe and start asking if our system is ready to use it responsibly
Rick Kimberly
While the data presented is compelling and the clinical distinctions between SGLT2 inhibitors are well-documented, it is imperative that clinicians and patients alike engage in shared decision-making grounded in individual risk profiles. The FDA’s decision to retain the warning while removing the boxed label reflects a nuanced understanding of benefit-risk calculus. Furthermore, the emphasis on pre-prescription screening - particularly ankle-brachial index assessment - represents a paradigm shift toward preventive, personalized care. It is not the pharmacological agent that is inherently hazardous, but rather the absence of structured clinical evaluation preceding its utilization
Terry Free
Oh wow so now we’re blaming the drug instead of the fact that people with diabetes don’t wear shoes? I mean if you’re numb in your feet and you’re walking around barefoot in your house then yeah you’re gonna get hurt - but that’s not INVOKANA’s fault that’s just dumb. And don’t even get me started on how everyone acts like losing a toe is the end of the world - it’s not like you’re losing your leg, it’s a toe. You still walk. You still live. You just can’t wear flip-flops anymore. Maybe if people stopped being dramatic and started checking their feet like they’re supposed to, this wouldn’t even be a thing
sagar patel
Canagliflozin causes amputation. Period. No debate. The numbers are clear. The FDA is compromised. The system is broken. I stopped taking mine after reading this. My feet are fine now. You’re welcome.
Linda B.
So let me get this straight - the same company that hid the opioid crisis is now telling us this drug is ‘safe enough’? And you believe them? The FDA removed the boxed warning because they were pressured by investors, not because the science changed. They’re still making billions. And now they want us to trust their ‘foot checks’ like that’s going to save us? I’ve seen what happens in clinics - five minutes, a quick glance, and then ‘you’re fine.’ If they really cared they’d mandate monthly podiatry visits covered by insurance. But they won’t. Because profit > people
Christopher King
Let’s be real - this isn’t about diabetes. This is about control. They want you to check your feet every day because if you’re busy obsessing over blisters you won’t ask why your insulin costs $300 or why your doctor only sees you for 8 minutes. They want you scared of your own body so you’ll keep taking the pill and never question the system. The toe amputation? It’s a distraction. The real amputation is your autonomy. Wake up. They’re not saving your life - they’re selling you a lifestyle subscription with extra steps
Bailey Adkison
Everyone’s acting like this is a new revelation. It’s not. The CANVAS trial was published in 2017. The risk was known. The FDA acted. The data was reviewed. The warning stayed. The drug stayed. That’s how medicine works - not by fear, but by evidence. If you have PAD and neuropathy and you’re still on canagliflozin? You’re not a victim of Big Pharma. You’re ignoring standard guidelines. This isn’t conspiracy. It’s clinical practice. Stop pretending you’re special because you read a Reddit post and now you know better than your endocrinologist