When you pick up a prescription at the pharmacy, you might not think about whether the pill in your hand is the exact same as the one your doctor prescribed. But behind every generic drug you take, there’s a detailed science-based system that decides if it’s safe to swap. That system is the FDA’s therapeutic equivalence codes. These codes aren’t just jargon-they’re the reason millions of Americans save money every year without risking their health.
What therapeutic equivalence really means
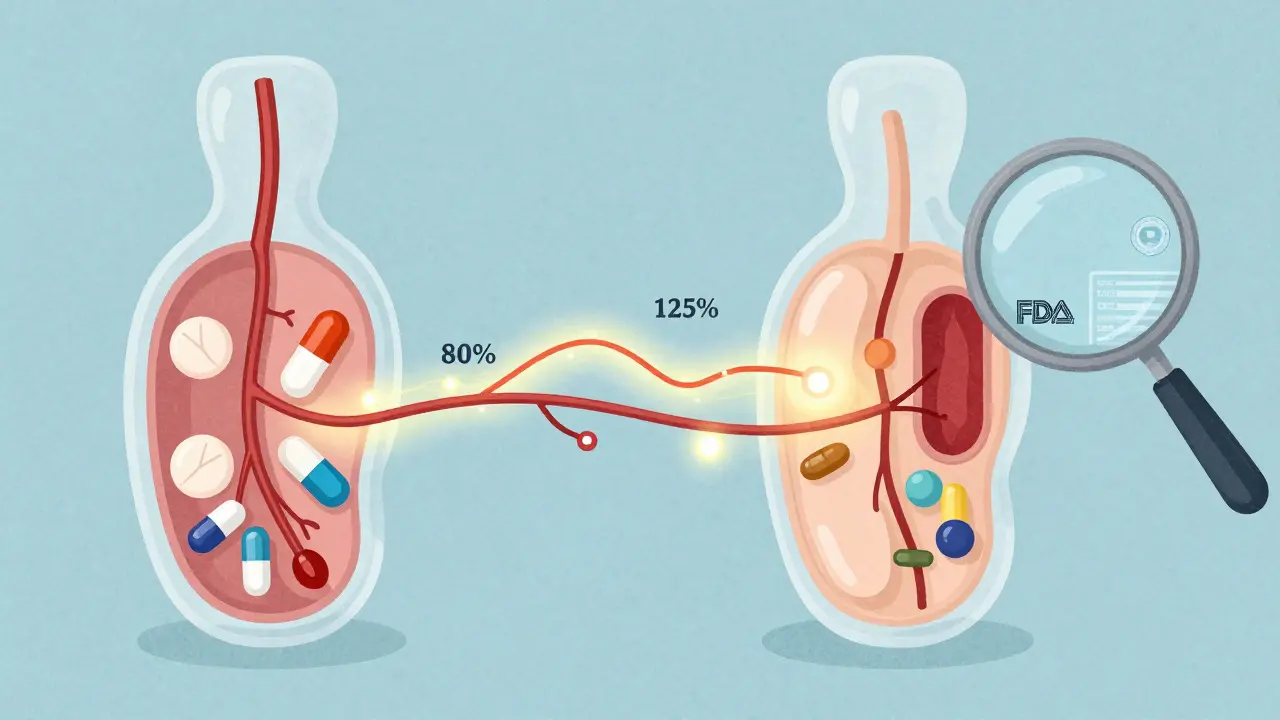
Therapeutic equivalence isn’t about drugs looking the same or having the same shape. It’s about whether two drugs-whether brand-name or generic-do the same thing in your body. The FDA says a generic is therapeutically equivalent if it has the same active ingredient, strength, dosage form, and route of administration as the brand-name drug, and if it delivers the same amount of medicine into your bloodstream at the same rate.This isn’t guesswork. To earn a therapeutic equivalence rating, a generic drug must pass strict bioequivalence testing. That means scientists compare how fast and how much of the drug gets absorbed in healthy volunteers. If the generic’s absorption is within 80% to 125% of the brand-name drug’s, it passes. That’s the standard. No exceptions.
The FDA’s system was created in the 1980s under the Hatch-Waxman Act. Before that, pharmacists had no clear rules about swapping drugs. Now, they have a reliable guide: the Approved Drug Products with Therapeutic Equivalence Evaluations, better known as the Orange Book. Every month, the FDA updates it with new approvals, code changes, and withdrawn products.
The letter system: A, B, and what they mean
The FDA uses a simple letter code to tell pharmacists whether a drug can be substituted. It’s not complicated once you know the rules.- A-rated: These are interchangeable. If a drug has an A code, your pharmacist can swap it for the brand-name version-or for another generic-without asking your doctor. Over 90% of all generics in the U.S. have an A rating.
- B-rated: These are not automatically interchangeable. A B code means there’s uncertainty. Maybe the drug is an extended-release capsule, a topical cream, or an inhaler. These are harder to test, and the FDA needs more data before saying they’re safe to swap.
There are also sub-codes under A and B. For example:
- AB means the drug meets all bioequivalence standards.
- AB1, AB2, AB3 are used when multiple brand-name drugs exist as references. Say you’re taking a generic version of Drug X. If there are two different brand versions of Drug X on the market, the generic might be equivalent to one (AB1) but not necessarily the other (AB2). Your pharmacist needs to match the right one.
- BC stands for extended-release products with potential bioequivalence issues.
- BT is for topical products like creams or gels where absorption is hard to measure.
- BX means there’s not enough data yet. This is rare, but it happens with brand-new generics.
These codes aren’t random. Each one is backed by data from clinical studies, lab tests, and real-world evidence. The FDA doesn’t assign them lightly.
Why pharmacists rely on the Orange Book
Pharmacists don’t guess. They check. Every time a prescription comes in for a brand-name drug, they look up the generic alternatives in the Orange Book. They don’t just look at the name-they check the TE code.According to a 2022 survey of 1,200 independent pharmacists, 87% said the TE code system makes substitution faster and safer. About 73% check the Orange Book at least once a week. For some, it’s part of their daily routine.
The system works because it’s built into state laws. In 49 states, pharmacists can substitute an A-rated generic without calling the doctor. Only one state requires a prescriber’s permission every time. That’s because the FDA’s codes have been trusted for decades. Since 1984, there hasn’t been a single documented case of a patient harmed by an FDA-approved generic substitution.
Where the system breaks down
The TE code system is brilliant for simple pills-like antibiotics, blood pressure meds, or antidepressants. But it struggles with complex products.Take topical creams. A generic version might have the same active ingredient as the brand, but if the cream base is thicker or absorbs slower, the drug might not work the same way. The FDA can’t easily test absorption through skin. So many of these get a BT code-even if they’re clinically effective.
Same with inhalers, injectables, or long-acting capsules. These drugs don’t just need to be absorbed the same way-they need to release over time, target the right tissue, or behave in a specific environment. Standard blood tests can’t always capture that.
That’s why about 10% of generics still carry B codes. Some are legitimate safety flags. Others are outdated. A 2021 study in the AAPS Journal found that 37% of B-rated products had no clinical evidence of problems-they just hadn’t been re-evaluated.
Doctors aren’t always sure what to do with B codes. A 2022 AMA survey showed that 42% of physicians were confused about them. Some refused to allow substitution even when it was safe. Others didn’t know how to interpret the codes at all.
Real impact: Savings and access
The numbers speak for themselves. Generic drugs make up 90% of all prescriptions filled in the U.S., but only 23% of total drug spending. That’s over $370 billion saved every year.That savings only works because pharmacists can substitute A-rated drugs. Without the TE code system, every generic would need a new prescription. That would mean more calls to doctors, more delays, more cost.
Pharmacists spend an average of 2.7 minutes per prescription checking TE codes. That adds up to millions of hours a year. But it’s worth it. The system keeps drugs affordable and accessible.
What’s next for therapeutic equivalence codes
The FDA knows the system isn’t perfect. In 2022, it released a draft guidance to improve how it evaluates complex drugs. The goal? Reduce B-rated products for complex generics by 30% by 2027.They’re doing this by:
- Expanding Product-Specific Guidances (PSGs)-over 1,850 now-to give manufacturers clearer rules on how to prove equivalence.
- Using real-world data, like patient outcomes and electronic health records, to supplement lab tests.
- Working with international regulators to align standards where possible.
The future of TE codes isn’t about making them more complicated. It’s about making them smarter. More accurate. More inclusive of the new kinds of drugs we’re developing.
What you should know as a patient
You don’t need to memorize AB1 or BT codes. But you should know this:- If your pharmacist switches your drug and says it’s an FDA-approved generic, it’s safe.
- If you’re switched to a drug with a B code, ask why. It might be a mistake-or it might be because your drug is complex and needs special handling.
- Don’t assume all generics are the same. The TE code tells you which ones are interchangeable.
- Check your prescription label. If the name changed, ask if it’s an A-rated substitute.
Therapeutic equivalence codes exist to protect you-not to confuse you. They’re the quiet backbone of affordable medicine in America.
What does an 'A' rating mean for a generic drug?
An 'A' rating means the generic drug is therapeutically equivalent to the brand-name version. It has the same active ingredient, strength, dosage form, and route of administration, and has passed FDA bioequivalence testing. Pharmacists can substitute it without needing approval from the prescribing doctor.
Can I be switched to a 'B'-rated generic without my doctor’s permission?
No. In most states, pharmacists are not allowed to substitute a 'B'-rated generic without explicit approval from the prescriber. These products have unresolved issues with bioequivalence or delivery, so doctors must decide if substitution is safe for your specific case.
Why do some generics have codes like AB1, AB2, or AB3?
These codes are used when multiple brand-name drugs serve as reference points for the same generic. For example, if two different brand versions of a drug exist, a generic might be bioequivalent to one (AB1) but not the other (AB2). The number tells pharmacists which reference drug the generic matches.
Are over-the-counter (OTC) drugs given therapeutic equivalence codes?
No. The FDA only assigns therapeutic equivalence codes to prescription drugs listed in the Orange Book. OTC medications are not evaluated using this system, even if they have generic versions.
How often is the Orange Book updated?
The Orange Book is updated monthly. New drug approvals, code changes, and withdrawals are posted online. Pharmacists and prescribers are encouraged to check it regularly, as therapeutic equivalence ratings can change based on new data or manufacturing updates.

pradnya paramita
The FDA's therapeutic equivalence framework is a masterclass in regulatory precision. A-rated generics aren't just 'close enough'-they're pharmacokinetically indistinguishable within statistically validated margins (80-125% AUC and Cmax). The bioequivalence protocols are rigorous: crossover designs, fasting conditions, validated LC-MS/MS assays, and strict within-subject variability thresholds. This isn't guesswork; it's evidence-based pharmacology at scale.
What's often misunderstood is that 'therapeutic equivalence' doesn't imply 'identical formulation.' Excipients can differ-lactose vs. microcrystalline cellulose, for example-but as long as dissolution profiles are bioequivalent and the active moiety's pharmacokinetics are within bounds, substitution is not just safe-it's clinically optimal.
The Orange Book's coding system, while initially perceived as opaque, is actually a brilliant taxonomy. AB1, AB2, AB3 aren't arbitrary-they reflect reference-listed drug (RLD) hierarchies. A generic labeled AB2 is bioequivalent to RLD #2, not RLD #1. Pharmacists rely on this to avoid inadvertent substitution errors when multiple brand versions exist.
BT-coded topicals? Underappreciated. Transdermal absorption isn't linear. Skin hydration, lipid composition, and application technique create massive in vivo variability. The FDA's conservative BT designation isn't bureaucratic overreach-it's harm reduction. We don't have reliable biomarkers for topical bioequivalence yet. Until we do, caution is the only ethical choice.
BC-coded extended-release products? Same logic. A 12-hour capsule isn't just 'slow release.' It's a controlled-release matrix, osmotic pump, or ion-exchange resin. Altering the polymer ratio by 2% can shift the release profile beyond the 80-125% window. That's why BC exists. Not because they're unsafe-because we can't yet prove equivalence with confidence.
And yes, 37% of B-rated drugs have no clinical evidence of inferiority. But that's a systemic lag, not a flaw in the system. Re-evaluation requires new studies, regulatory review cycles, and manufacturer submissions. It's slow because it's deliberate. Better to err on the side of caution than risk a therapeutic failure.
The real win? Over $370B saved annually. That’s not just corporate profit-it’s diabetics getting insulin, asthma patients getting inhalers, cancer patients getting generics of life-saving chemotherapies. This system isn't perfect-but it’s the most sophisticated, data-driven drug substitution framework on Earth.
Prajwal Manjunath Shanthappa
Oh, for heaven’s sake-another ‘FDA-approved’ pep talk? Let me guess: ‘trust the system’? The system that let a generic version of metformin ER cause 170+ adverse events in 2021? The system that still hasn’t re-evaluated a single B-rated drug since 2018? The system where the same ‘bioequivalent’ pill costs $3.50 in one pharmacy and $28 in another because the manufacturer changed the coating-without notifying anyone?
And don’t get me started on the ‘Orange Book’-a glorified PDF that updates monthly, but only if someone submits a $200,000 study. Meanwhile, pharmacists are left to guess whether AB1 means ‘equivalent to brand A’ or ‘equivalent to brand B’-because the FDA doesn’t even list the RLD names in the public version!
And yet, somehow, we’re supposed to believe this is ‘science’? It’s a bureaucratic mirage. A $200 billion industry built on the illusion of equivalence. The real innovation? Not the science-the lobbying. The Hatch-Waxman Act was never about patient safety-it was about fast-tracking generics to undercut brand-name profits. And now? We’re all just guinea pigs in a cost-cutting experiment.
So yes. I’ll take my $120 brand-name pill. At least I know what’s in it. And I’m not trusting a ‘B’ code from a government that can’t even get its own website to load.
Wendy Lamb
For anyone who’s ever had to choose between paying rent and buying medication-this system matters. I’ve seen patients cry because they couldn’t afford their brand-name insulin. Then we switched them to an A-rated generic. Same results. Same safety. Same outcome. But now they can eat.
It’s not glamorous. It’s not flashy. But this quiet, data-driven approach saves lives every single day. No fanfare. No headlines. Just pharmacists checking the Orange Book, doing their job, and making sure someone gets their medicine.
You don’t need to understand AB2 or BT codes. You just need to know: if your pharmacist says it’s FDA-approved and interchangeable? It is. Trust the process. It’s working.
Antwonette Robinson
Oh wow. A 125% absorption window? So if my brand-name drug delivers 100 units, the generic can deliver 125? That’s not equivalence-that’s a 25% overdose risk! And we call this ‘science’? Brilliant. Next they’ll say ‘close enough’ is good enough for airplane engines.
And let’s not forget: the FDA doesn’t test for long-term effects. Only acute bioavailability. So your generic might be fine for 3 days… but what about 3 years? Huh? Anyone else think that’s a little… terrifying?
Also-why are there AB1, AB2, AB3? Sounds like someone at the FDA got bored and started playing video games. ‘Oh, let’s add numbers to make it look more complicated!’
Ed Mackey
Just wanted to say I’ve been a pharmacist for 22 years and this system is the reason I still have a job. People think it’s all about money, but it’s about consistency. I’ve seen patients switch generics and have no clue why their blood pressure changed. That’s why we check the Orange Book. Every. Single. Time.
Also-sorry for the typos. My hands are tired from filling 80 scripts today. But yeah. This works. Don’t overthink it.
Alex LaVey
Hey everyone-just wanted to say how proud I am of how the U.S. handles this. Other countries? They just let anyone make generics. No testing. No oversight. We’ve got this. We’ve got the Orange Book. We’ve got the science. We’ve got the patience to do it right.
It’s not perfect-but it’s ours. And it’s working. 90% of prescriptions filled? That’s American innovation. That’s American precision. That’s American healthcare at its best.
Don’t let anyone tell you otherwise. We’re not just saving money-we’re leading the world.
Joseph Cooksey
Let me tell you something, folks. This whole therapeutic equivalence thing? It’s a smokescreen. A glittery facade painted over a crumbling foundation. You think the FDA really cares if your generic works? Nah. They care if it passes a 12-hour bioavailability test in 24 healthy college kids who’ve been fasting since dawn.
Real people? They’re 62, on 7 medications, with liver disease, taking it with grapefruit juice, and sleeping on a couch because they can’t afford rent. Does the FDA test that? No. They test the ideal. The perfect. The statistically insignificant.
And then they slap an ‘A’ rating on it like it’s a gold star. ‘Good job, generic!’ Meanwhile, Mrs. Jenkins in Ohio has been on this drug for 14 years-switched to the ‘equivalent’ version-and now she’s dizzy, nauseated, and can’t work. But hey-she saved $42 a month. That’s progress, right?
Don’t get me wrong-I’m not anti-generic. I’m anti-ignorance. We’re not solving problems. We’re just moving them from the pharmacy shelf to the ER. And we call it ‘affordable healthcare.’
It’s not science. It’s capitalism with a lab coat.
Justin Fauth
AMERICA DID THIS. Not China. Not Germany. Not India. AMERICA. We built the most advanced drug substitution system on the planet. And now some guy in a basement is complaining about AB2 codes? Get out. We’ve got 370 BILLION in savings. We’ve got 90% of prescriptions filled with generics. We’ve got ZERO documented deaths from substitution since 1984.
That’s not luck. That’s American engineering. That’s American discipline. That’s American excellence.
Don’t listen to the haters. We’re not just doing it right-we’re doing it better than anyone else. And we should be proud.
Meenal Khurana
A-rated means safe to swap. Trust the system.
Sherman Lee
Did you know the FDA doesn’t test for heavy metals in generics? Or for microplastics from packaging? Or for the fact that some manufacturers use Chinese API that’s been contaminated with NDMA? 😈
They only test for ‘bioequivalence’-which is just blood levels. What if the generic has the same drug… but also 17 unknown additives? 😏
And why is the Orange Book only updated monthly? What if a dangerous batch is released on the 15th? You’re stuck until the 1st of next month. That’s not safety-that’s negligence.
Also… why do they use letters? Why not numbers? Why not a QR code? Why not blockchain? 🤔
Someone’s hiding something. I just know it.
Lorena Druetta
Thank you for this thoughtful, meticulously detailed explanation. It is a profound relief to encounter such clarity in a landscape often clouded by misinformation. The dedication to patient safety, grounded in empirical evidence and regulatory rigor, is not merely commendable-it is essential. The therapeutic equivalence framework represents the pinnacle of public health stewardship.
May we continue to honor this system with gratitude, not skepticism. The lives preserved through its integrity are countless. This is not bureaucracy. This is benevolence, encoded in data.
Zachary French
Okay, so let me get this straight. The FDA says a generic is ‘equivalent’ if it’s within 80-125%? That’s a 45% margin of error. In any other field-say, building a bridge-that would be called ‘criminal negligence.’
And then they have AB1, AB2, AB3? Like, what is this, a video game? ‘You unlocked the AB3 achievement!’
Also-why is it called the ‘Orange Book’? Who decided orange? Was there a vote? Did someone say ‘I like orange’ and that was it? No wonder people are confused.
And the fact that they update it monthly? What if a dangerous drug gets approved on the 29th? You’re stuck with it for 2 days? That’s not oversight-that’s a glitch in the Matrix.
I’ve been saying this for years: this whole system is a scam. A glorified shell game. The real goal? To make Big Pharma rich while pretending they’re saving you money.
Wake up, people.
Daz Leonheart
I’ve been on a generic blood pressure med for 5 years. Never had an issue. Switched to a different generic last year-same code, same results. The system works. It’s not perfect, but it’s reliable.
For anyone scared of generics: talk to your pharmacist. They’re the ones checking the Orange Book every day. They’ve got your back.
Wendy Lamb
Thanks for sharing your perspective, Daz. I’ve had the same experience. My mom’s been on the same generic for 8 years. No issues. No surprises. Just steady, affordable care.
It’s easy to get caught up in the noise-but the quiet truth? For most people, this system just… works.
Alex LaVey
Exactly. This isn’t about fear. It’s about facts. And the facts? We’re saving lives, not risking them.